MEDOMICS SARS-COV-2 ANTIGEN TEST KIT (LFIA) 20 TEST (PROFESSIONAL)
$ 200.00 $ 156.00
$ 200.00


MEDOMICS SARS-COV-2 ANTIGEN TEST KIT (LFIA) 20 TEST (PROFESSIONAL)
Medomics SARS-CoV-2 Antigen Test Kit (LFIA) is used for in vitro detection of SARS-CoV-2 in nasopharyngeal swab, throat swab, nasal swab samples.
● Packing size: 20 tests/kit
● Specimen type: Nasal swabs, Throat swabs, BALF
● Instrumentation: not required
● Detection time: 15-20 minutes
● Storage: Room temperature (2-30°C)
● Shelf life: 24 months
Product Code : SARS-COV-2
30-day money-back guarantee
Free Shipping over $150 spent in Brisbane Metro *. All Prices Excluding GST*
I am (select all that apply)
SARS-CoV-2 antigen Test Kit (LFIA) Instructions For Use
Product Name |SARS-CoV-2 antigen Test Kit (LFIA)
Package specifications| Types: VI Test card: 1pc/bag Kit: 1 pc/box, 20 pcs/box, 50 pcs/box, 100 pcs/box
Intended use
Medomics SARS-CoV-2 antigen Test Kit (LFIA) is used to qualitatively detect SARS-CoV-2 in human samples in vitro.
Coronavirus (CoV) belongs to the order Nidovirales under the Coronaviridae family with 4 genera:a, β, V and δ.
The a and β genera are only pathogenic to mammals, while V and δ genera mainly causes bird infections. CoV is mainly transmitted through direct contact with secretions or through aerosols and droplets. There is also evidence supporting fecal-oral transmission.
7 kinds of human coronaviruses (HCoV) that cause human respiratory diseases have been identified so far, including:
HCoV-229E, HCoV-NL 63, HCoV-OC43, HCoV-HKU1, SARS -CoV, MERS-CoV and SARS-CoV-2. SARS-CoV-2 is one of the most contagious viral pathogens that causes human respiratory tract infections (RTI). Currently, the patients infected by novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days.
The clinical manifestations include fever, fatigue, cough and other symptoms, accompanied by dyspnea, which can rapidly develop into life-threatening severe pneumonia, respiratory failure, acute respiratory vesicle syndrome, septic shock, multiple organ failure, and severe metabolic acid-base imbalance.
Test Principle
Medomics SARS-CoV-2 antigen Test Kit (LFIA) detects the novel coronavirus with colloidal gold mmunochromatography using a double antibody sandwich assay. The test cassette contains (1) colloidal gold-labeled anti-SARS-CoV-2 antibody, (2) one detection line (T line) and one quality control line(C) fixed on a nitrocellulose membrane. T is fixed with anti- SARS-CoV-2 antibody for detecting the novel coronavirus. The quality control antibody is fixed on the C line.
When the appropriate amount of test sample treated with lysis buffer is added to the sample well of the test cassette,the sample will move forward along the test strip via capillary action. If the sample contains the novel coronavirus antigens and concentration of antigens is higher than the limit of detection, the antigen will bind to the colloidal gold-labeled anti-SARS-CoV-2 antibody. The immune complex will be captured by the anti-SARS-CoV-2 antibody immobilized on the membrane, forming a red T line and indicating a positive result for the novel coronavirus. If the sample contains no novel coronavirus antigens or the antigens concentration is lower than the limit of detection, a negative result is displayed.
Additionally, the test cassette also contains a quality control line(C). Regardless of what antigens are present the C line should appear to indicate that the sample has been transported properly through the membrane. If the C line does not appear it indicates that the test result is invalid and the sample is required to retest.
line should appear to indicate that the sample has been transported properly through the membrane. If the C line does not appear it indicates that the test result is invalid and the sample is required to retest.
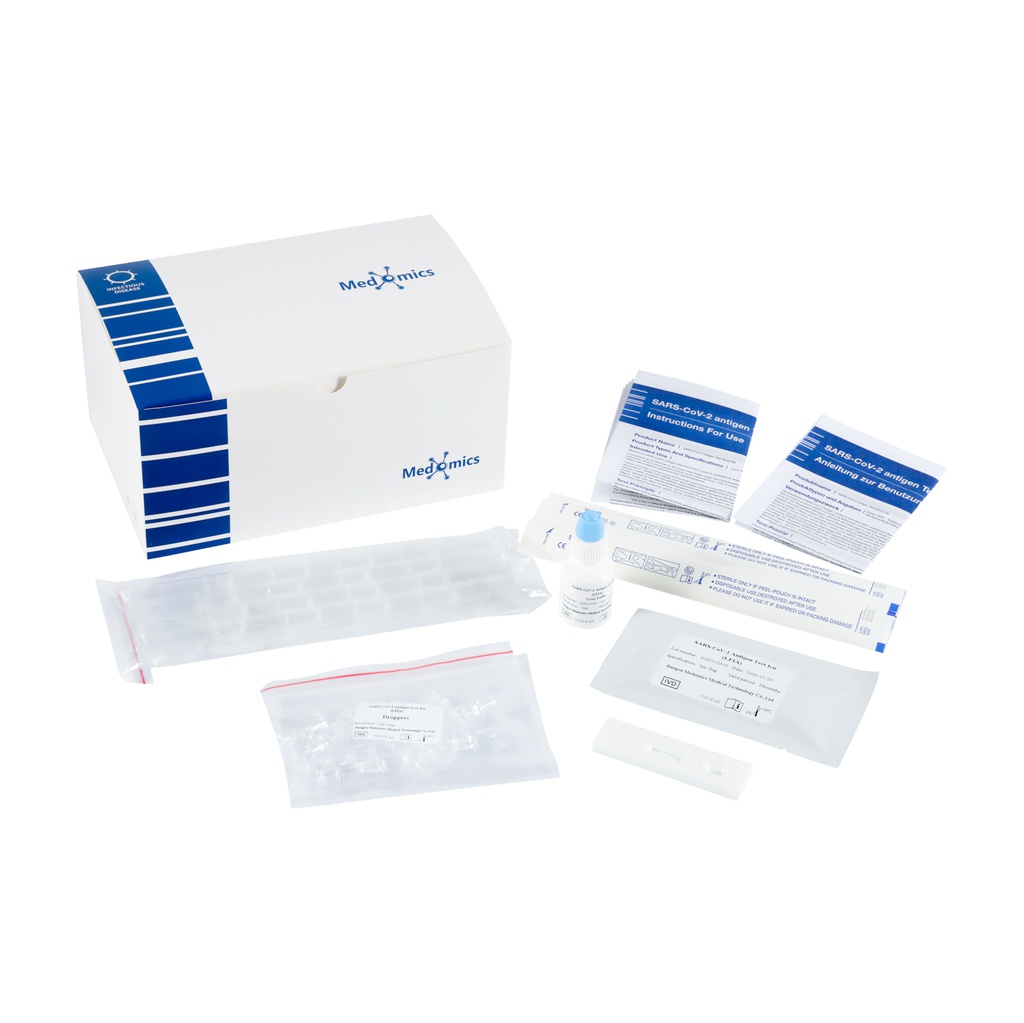
Contents of the Kit
Type | test kit contains test cassettes, lysis buffer, sampling tubes, droppers and instructions for use. Type II test kit contains test cassettes, lysis buffer, sampling tubes, droppers, sterile swabs and instructions for use.
●Test cassette: contains a test strip and a plastic cassette casing. The test strip contains: colloidal gold-labeled anti-SARS-CoV-2 antibody, nitrocellulose membrane (C-line fixed with goat anti-rat IgG polyclonal antibody, and T-line fixed with anti- SARS-CoV-2 antibody).
Warnings and Precautions
● This test kit is used for in vitro auxiliary diagnosis only.
● This test kit should be used by qualified personnel with professional experience or proper training.
● This test kit should be used within 1 hour after opening the package. The test cassette should not be used if being wet or polluted.
● Proper protection should be taken during testing to avoid splashing when adding sample.
● Dispose of all used or damaged test cassettes, capillary samplers, or other kit components as biohazardous materials.
● Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
Storage Instructions
The test kit should be stored away from direct sunlight at room temperature (2 to 30°C) and has a shelf-life of 12 months. The container should be protected from light after being opened. Do not freeze.
Sample Requirements
Sterile swab is recommended for collecting samples.
● Nasal secretion collection: Insert the swab into the nasal cavity where there is the most secretion, gently spin and push the swab forward until blocked by the turbinate (about 2.0cm-2.5cm from the nostri). Then rotate and rub the swab on the cavity wall 3 times before taking it out.
● Throat secretion collection: Insert the whole swab completely into the throat from the mouth, centering on the throat wall and the reddened area of the palate tonsil, wipe both sides of the pharyngeal tonsil and posterior pharyngeal wall with moderate force. Try to avoid the tongue before taking it out.
Sample should be treated with lysis buffer provided in this kit as soon as possible after collection. If the sample cannot be processed immediately, it should be stored immediately in a dry, sterilized and strictly sealed plastic tube. It can be stored at 2°C-8°C for 8 hours. Long term storage is available at -70°C.
Test Procedure
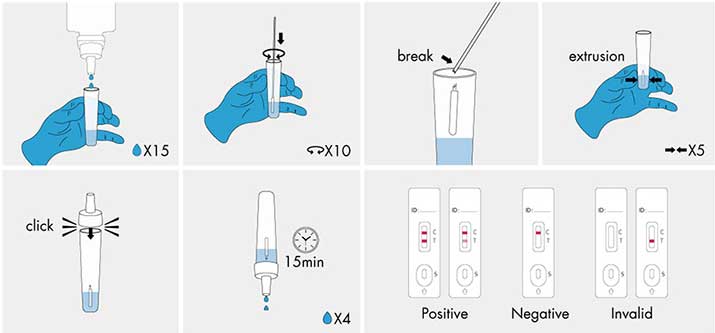
Do not open pouch until ready to use. Prep necessary materials: Sampling tube | Lysis buffer solution | Dropper | Test cassette marked with patient ID or sample number.
1| Sampling: Vertically add 15 drops (approximately 375 ul) lysis buffer into the sampling tube. Insert the swab (after collection) into the solution. Rotate the swab against the inner tube wall 10 times and break the swab handle, then squeeze the swab from the outer tube wall 5 times to completely dissolve the sample in the solution and cover the tube with the dropper.
2 | Test procedures: Open the aluminum foil bag, take out the test casstte and lay it on a clean flat surface. Add 4 drops (approximately 100 ul) processed sample extract into the sample well.
The result should be observed within 15-20 minutes. Results observed after 20 minutes are of no clinical significance.
Test Method Limitations
● The accuracy of the test is dependent on the quality of the sample. Improper sampling and storage, using expired samples or repeated frozen-thawed samples can affect the test result. Test results can also be affected by temperature and humidity.
● Negative results may be caused by low concentrations of SARS-CoV-2 antigens in the sample and therefore cannot completely rule out the possibility of infection.
● Some medication (e.g. high concentrations of over-the-counter (OTC) or prescription medication such as nasal spray) in the collected samples may interfere with the test result. Please perform the test again if the result is in doubt.
● This product is only for qualitative testing and the specific concentration of each indicator must be measured using other quantitative methodologies.
● The results of this test are for clinical reference only and should not be the only basis for diagnosis. Results should be used in combination with clinical observations and other testing methods.
Display of Results/Expected Values
● Negative result: If only the quality control line (C) appears and the detection T line is not visible, the sample contains no novel coronavirus antigens or the concentration of the antigen is lower than the limit of detection and the result is negative.
● Positive result: If both the quality control line (C) and the detection line T appears, then the novel coronavirus has been detected and the result is positive.
● Invalid result: If the C-line does not appear, the result is invalid and a new test must be performed again.
Note: The color intensity of the T-line is related to the concentration of SARS-CoV-2 antigens contained in the sample, and the result should be determined by whether the Online is coloured or not regardless of the color intensity.
Product performance index
● Appearance: complete outer packing box; aluminium foil bag with no damages; the test cassette should be neat, complete, and free of damage and contamination; the plastic cassette should be firmly assembled; desiccant with no damage; lysis buffer with no sediment, flocculent suspended solid or leakage.
● Verification with external control should meet the following requirements
1| Coincidence rate of negative control: The test results of 10 negative controls should all be negative (10/10).
2 | Coincidence rate of positive control: The test results of 10 positive controls should all be positive (10/10).
● Repeatability
1 | Repeatability within lots
Take kits from the same lot, and repeat the test with weak-positive quality control 10 times. The reaction results should be consistent, and the color rendering should be uniform (10/10).
Take kits from the same lot, and repeat the test with strong-positive quality control 10 times. The reaction results should be consistent, and the color rendering should be uniform (10/10).
2| Repeatability across lots
Perform the test for the kits from three different lots with weak- positive quality control. The reaction results should be consistent, and the color rendering should be uniform (30/30).
Perform the test for the kits from three different lots with strong-positive quality control. The reaction results should be consistent, and the color rendering should be uniform (30/30).
● Analytical Performance
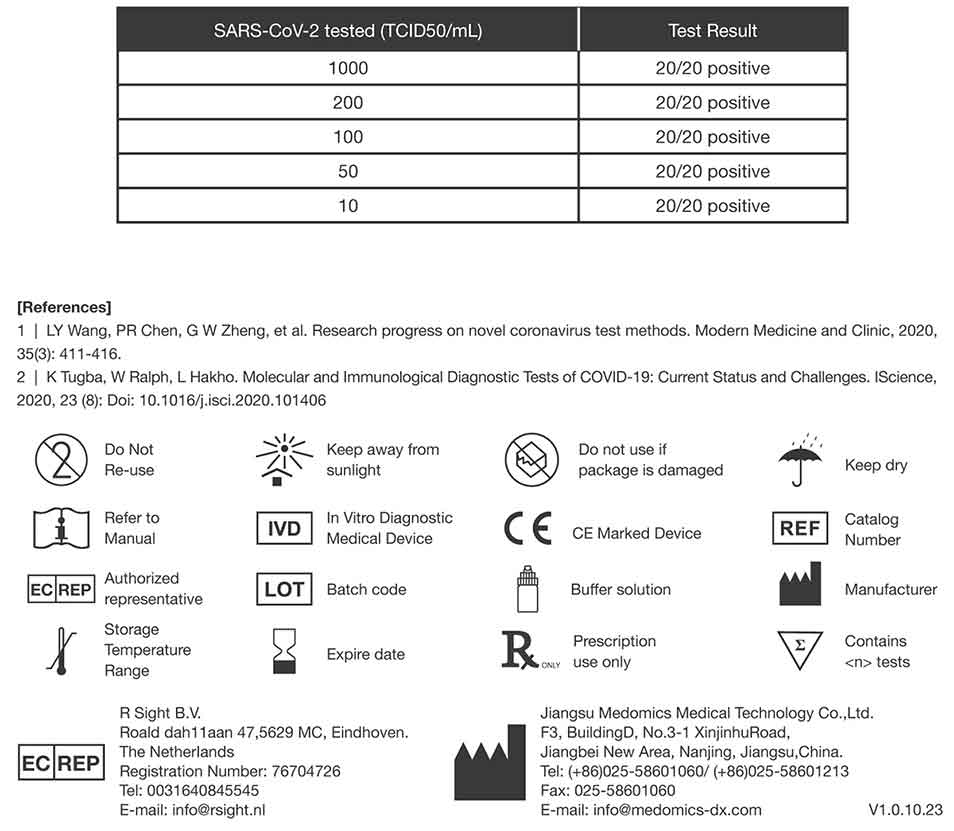
Sensitivity (Limit of Detection- LOD)
Limit of Detection (L .oD) studies determined the lowest detectable concentration of SARS-CoV-2 at which 100% of all (true positive) replicates test positive. Dilute the SARS-CoV-2 virus with lysis buffer to a final concentration gradient of 10, 50, 100, 200, 1000 TCID50/mL.